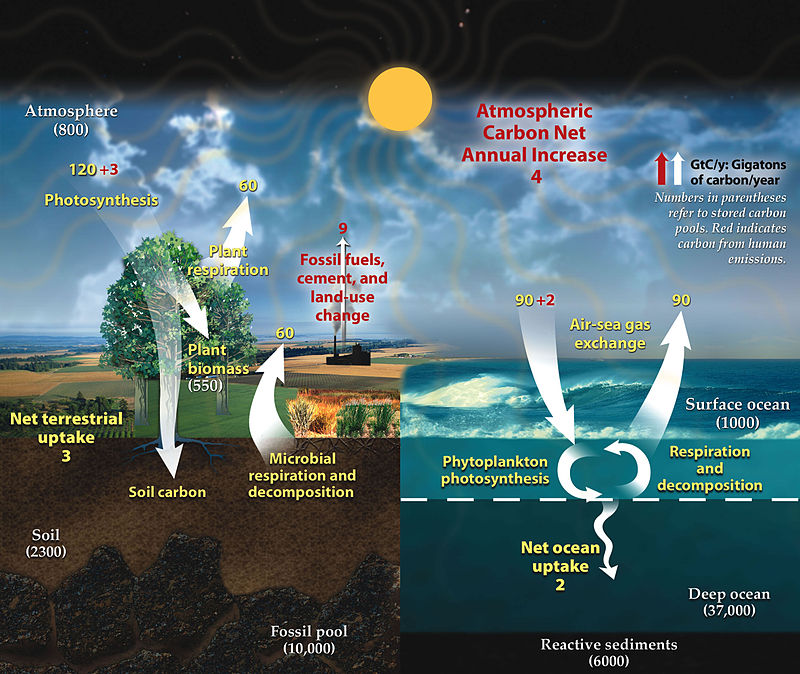
The Carbon cycle
Carbon is the main component of biological compounds as well as a major component of many minerals.The carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life.
Using energy from the Sun, both plants and plankton combine carbon dioxide (CO2) and water to form sugar (CH2O) and oxygen. The chemical reaction looks like this:
CO2 + H2O + energy = CH2O + O2
On the reverse sequence of events, plants break down the sugar to get the energy they need to grow. Animals eat the plants or plankton, and break down the plant sugar to get energy. Plants and plankton die and decay (are eaten by bacteria) at the end of the growing season. Fire consumes plants. In all these cases, oxygen combines with sugar to release water, carbon dioxide, and energy. The basic chemical reaction looks like this:
CH2O + O2 = CO2 + H2O + energy
Human activity has impacted the natural carbon cycle. The natural carbon cycle has severely interrupted by clearing the natural vegetation for agriculture and cultivation. This has resulted in decline in soil organic matter levels, of which 58% is carbon. This is mainly due to the reduction of organic residues in the soil and increased aeration (oxidation) of the soil brought about by cultivation. Loss of soil organic carbon is one of the principal signs of land degradation.
When land is degraded, soil carbon can be released into the atmosphere, along with nitrous oxide, making land degradation one of the biggest contributors to climate change. An estimated two-thirds of all terrestrial carbon stores from soils and vegetation have been lost since the 19th century through land degradation.
Not all human activity, however, results in the decline in soil organic matter levels. By replacing relatively unproductive native vegetation with productive pasture species, substantial increases in soil organic matter levels can be obtained (Johnston, A.E. 1980).
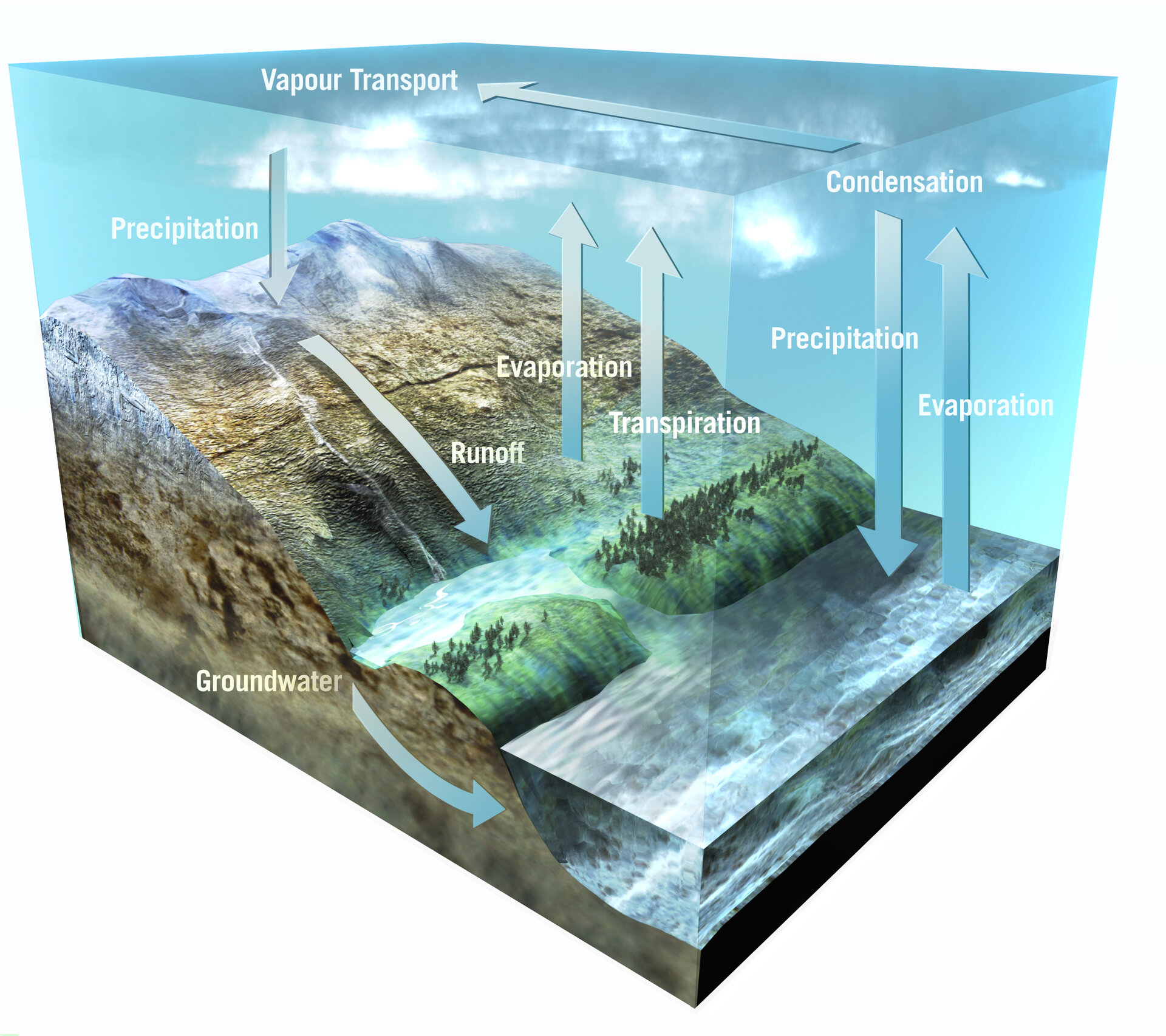
The Water cycle
Water is a renewable resource that belongs to a gigantic cycle, the hydrologic cycle. It is evaporated from the earth and the sea into the atmosphere. The energy required for evaporation is supplied by the sun. In the atmosphere, water is transported by the wind in the form of vapor, until it finally returns to the earth in the form of precipitation.
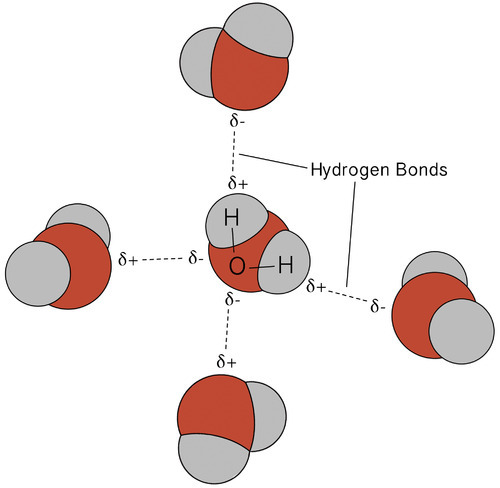
As water is essential for life and an understanding of its basic properties is essential to the study of soils and plants. Water molecules have an asymmetrical arrangement of hydrogen and oxygen atoms. The H-O-H bond is set an an angle of 105 and this means that the two hydrogen atoms are located at one side of the molecule, with the oxygen atom at the other. The water molecule have a slightly positive charge with the side of the hydrogen atoms, while the side with the oxygen atom has a slight negative charge.
The polar nature if water molecules causes them to orientate themselves in the liquid or solid in such a way that the oxygen atom of one molecule is closest to the hydrogen atom of another.This linkage in between water molecules in called hydrogen bonding. It is an important property because it results in the attraction of water molecules for each other (cohesion) and the attraction of water for other surfaces(adhesion). Adhesion and cohesion provide the soil with ability to store water. Soil which is rich in soil organic matter and soil carbon has a better ability to hold water.
The Phosphorus cycle
In grazing pastures, a large proportion of the phosphate taken up by the pasture and ingested by the grazing animal is returned to the soil as dung or as plant residues. Above-ground losses of phosphate from the cycle, accounting for approximately 20%-35% of the phosphate taken up by the pasture, are mainly in the form of animal products and dung distributed to unproductive areas like yards.
A large proportion of the phosphate in both dung and plant residues (70%-80%) is water soluble and in the course of the year is returned to the soil solution. Phosphate released by enzymatic breakdown from decaying organisms (mineralization) may be either re-immobilized back in the biomass or be taken up by plant roots. Biomass helps to maintain availability of phosphate to plants and may protect phosphate against inorganic fixation reactions.
Nematodes and fungi are examples of soil organisms that make Phosphorus more available for plants.

The Nitrogen cycle
The Nitrogen occurs in many forms in nature, with very large amounts being found both in the earth’s crust (18 x 10^15 tonnes) and in the atmosphere (3.8 x 10^15 tonnes of Nitrogen gas, N2).
Atmospheric N2 must be fixed by soil organisms like Nitrogen fixing bacteria before it can be used. Even most Nitrogen in soil is unavailable to plants as it is part of the soil organic matter fraction. Plant nutrition depends on efficient cycling of N from unavailable soil organic forms to available mineral forms, such as nitrate (NO3-) and ammonium (NH4+).
In pastoral grazing, the animal returns large amounts of N to the soil plant system in the form of urine and dung. The transfer of N from one form to another within the soil/plant/animal/atmosphere system is referred to a the Nitrogen cycle.

